Cannabis Compliance Testing
 Rectangle 3019
Rectangle 3019 The legalization of California’s medical and adult-use cannabis market has led to the daunting task of implementing statewide regulations for the largest cannabis market in the world. The Department of Cannabis Control (DCC) is the lead regulatory authority responsible for licensing and overseeing commercial businesses involved in each step of the supply chain.
Testing regulations require all batches of finished products to be sampled and tested by an accredited third party laboratory to ensure safety and dosing accuracy.
As of December 31, 2018, all cannabis and cannabis products are required to be in compliance with Phase 3 testing regulations.
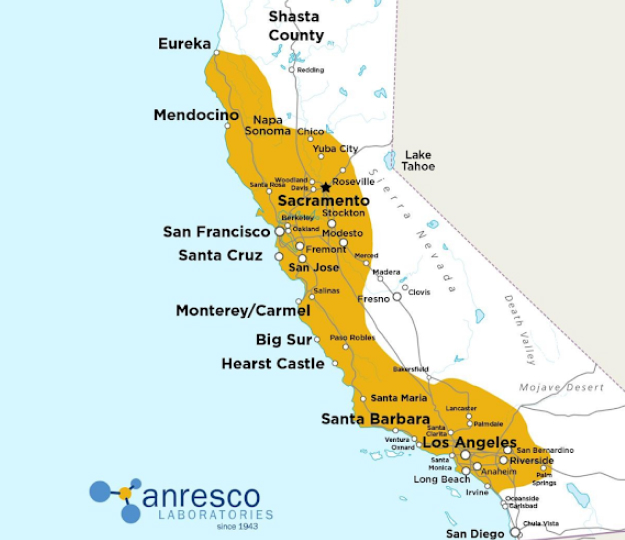 AnrescoLab California locations
AnrescoLab California locations Available Throughout California
The highlighted areas are the locales we already have routine pickups scheduled, though we are certainly willing to expand that radius as needed by our clients.
Committed to
Provide Exceptional Assistance
Anresco Laboratories boasts a dedicated customer support team committed to providing exceptional assistance to clients. With a focus on addressing inquiries related to client portals, order processing, result interpretation, and formulation consultations, our team goes the extra mile to ensure seamless communication and support for all aspects of our clients’ needs.