Compliance Begins for new FDA Nutrition Facts Labels
January 1, 2020 marks the compliance deadline of new FDA nutritional labeling requirements for companies with more than $10 million in annual food sales. While smaller companies have an additional year to comply, all imported foods must also meet these requirements.
On December 31, 2019, the FDA issued a final guidance document to help food manufacturers comply with the new regulations and address questions and concerns relating to the previous two final rules. The final guidance document primarily addresses “serving sizes of foods, including single-serving foods and other foods that can reasonably be consumed at one eating occasion and require dual-column labeling.”
In an FDA press release, the agency noted that enforcement will not be a priority for the first six months of 2020. Instead, the FDA intends to focus their efforts on assisting food manufacturers come into compliance.
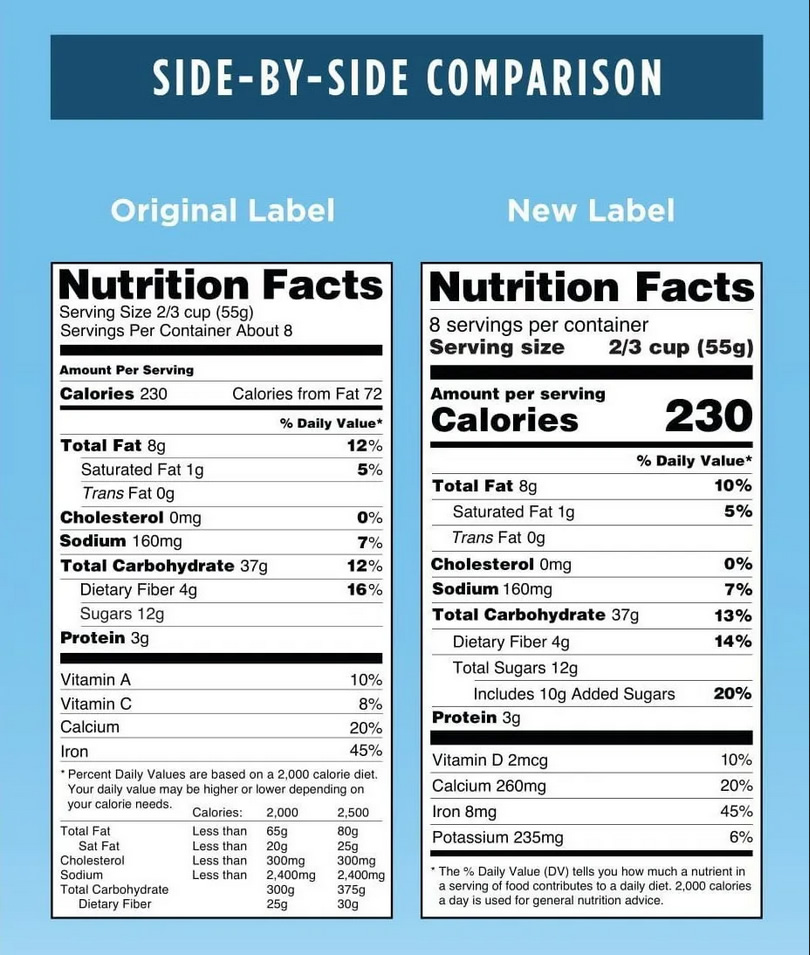
Consumers are likely to have already seen the new FDA nutrition facts label on food packages. Significant changes include the removal of vitamin A and C from the mandatory label and the addition of vitamin D, potassium, and added sugar. In addition, calories will now be displayed in a larger bold font and recommended serving sizes of various foods have been adjusted to better reflect what Americans consume.
Text of the FDA’s final rules can be found below:
Final Rule: Revision of the Nutrition and Supplement Facts Labels
Anresco Laboratories is family owned and provides comprehensive analytical testing services to food and cannabis industries. Contact us for a quote to update your nutrition facts label in accordance with new FDA requirements.