Nutritional Labeling
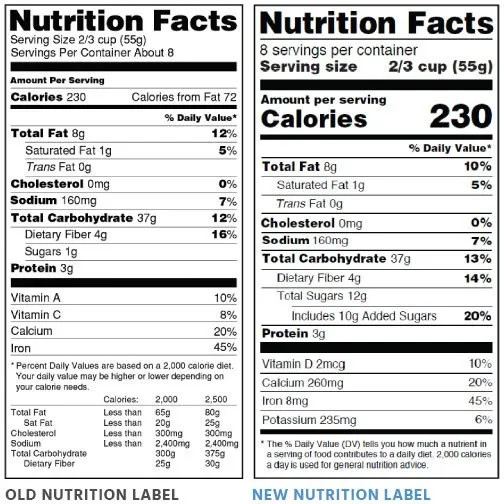 Nutrition label
Nutrition label Anresco has offered nutritional and dietary supplement labeling services since 1973, helping thousands of companies comply with FDA/USDA labeling regulations.
The Nutrition Labeling and Education Act (NLEA) of 1990 authorized the U.S. Food & Drug Administration to require nutrient labeling on almost all foods sold domestically. Since then, the FDA has issued additional rules requiring nutritional labeling for foods in vending machines (November, 2014 ), at “chain” restaurants and grocery stores (December, 2014), as well as new requirements for packaged foods (July, 2015).
How are Labels Generated?
Database Calculation
Most labels can be generated using a database calculation method. We use a computerized program called Genesis R&D to calculate the nutrient values based on the recipe we’re provided. With this method, no physical sample needs to be submitted to us.
Chemical Analysis
For some products, analytical testing must be performed on a physical sample in order to obtain accurate nutrient values. This includes products that are fermented, fried, marinated, brewed, or brined. Lab testing offers greater accuracy as results reflect the actual product on the market.
Nutrition Facts Labeling Requirements
The Mandatory Label Information includes:
- Calcium
- Protein
- Added Sugars
- Calories
- Potassium
- Sugars
- Carbohydrates
- Saturated Fats
- Total Fat
- Cholesterol
- Serving Size
- Trans Fatty Acids
- Dietary Fiber
- Servings Per Container
- Vitamin D
- Iron
- Sodium
Analyses Included upon Request:
- Monounsaturated Fats
- Other Carbohydrates
- Polyunsaturated Fats
- Ingredients List (available for database labels only)
Voluntary Analyses Include:
- Vitamin A
- Aluminum
- Phosphorus
- Vitamin C
- Copper
- Selenium
- Vitamin E
- Iodine
- Tin
- Vitamin K
- Magnesium
- Zinc
- Thiamin
- Manganese
- And More…
- Riboflavin
- Molybdenum
- Niacin
- Nickel
Dietary Supplement Facts Labeling Requirements
The FDA defines a dietary supplement as the following:
-It contains a vitamin, mineral, botanical, amino acid, enzyme, dietary supplement, or some combination of these things.
-The active ingredient isn’t a medicine used to treat an illness or an effect of an illness.
-The product is intended for ingestion.
-The product isn’t represented as a conventional food or a sole item of a meal or a diet.
Manufacturers are required to select the correct label type based on intent. Any health claims should be made with caution.
While the Supplement Facts label looks similar to the Nutrition Facts label, only the active ingredients are required to be labeled.
Anresco has expertise in validating the following common ingredients:
- Amino Acids
- Inositol
- Vitamin A (Beta-carotene)
- Androstenol
- Iron
- Vitamin A (Retinol)
- Beta-sitosterol
- Magnesium
- Vitamin B1 (Thiamine)
- Caffeine
- Manganese
- Vitamin B2 (Riboflavin)
- Calcium
- Melatonin
- Vitamin B3 (Niacin)
- Calories
- Piperine
- Vitamin B5 (Pantothenic Acid)
- Carbohydrates
- Potassium
- Vitamin B6 (Pyridoxine)
- Cholesterol
- Protein
- Vitamin B7 (Biotin)
- Coenzyme Q10
- Quercetin
- Vitamin B9 (Folic Acid)
- Copper
- Sodium
- Vitamin B12 (Cyanocobalamin)
- Creatine Monohydrate
- Sugar Profile
- Vitamin C
- Dietary Fiber
- Testosterone
- Vitamin D
- Fatty Acids (including omegas)
- Tin
- Vitamin E
- Free Amino Acids
- Trans-Resveratrol
- Zinc
Enlist our expertise to help you navigate the changing regulatory requirements of the food and dietary supplement industries.